Healthcare Professionals
This page is intended for healthcare professionals.
GvHD : Pathology & Epidemiology
Graft-versus-Host Disease (GvHD) is one of the potential complications following an allogeneic hematopoietic stem cell transplantation (HSCT). It is estimated that 30 to 50% of patients develop GvHD after an allogeneic HSCT.
There are two types of GvHD, distinguished notably by the timing of symptom onset: symptoms occurring within 100 days after transplantation are classified as acute GvHD, while those appearing later are considered chronic GvHD. The symptoms also differ between these two forms.
- Chronic GvHD presents with more diffuse symptoms. It can affect the skin, eyes, mouth, muscles, and gastrointestinal tract, and may lead to infections and breathing difficulties.
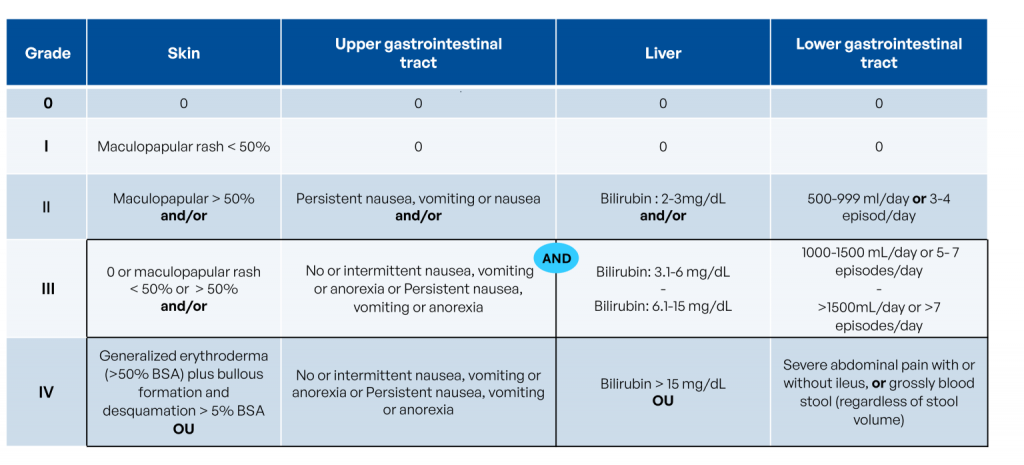
- Acute GvHD, on the other hand, primarily targets three organs: skin, liver, and gastrointestinal tract. Acute GvHD can be severe and rapidly fatal. It is one of the most feared complications following allogeneic HSCT and generally has a poorer prognosis.
During an allo-HSCT, despite careful donor selection based on HLA matching, the graft can attack the patient’s tissues, recognizing them as foreign.
The severity of GvHD is graded on a scale from 0 (no reaction) to 4 (severe reaction):
The Gut Microbiome: An Innovative Approach in the Management of Blood Cancers
In hematologic malignancies, the gut microbiome plays a crucial role in modulating immune response and enhancing treatment efficacy. Dysbiosis caused by cancer itself, chemotherapy, or antibiotics may trigger systemic inflammation and impact patient outcomes. Emerging evidence shows that microbiota restoration can improve treatment tolerance, lower the risk of complications such as graft-versus-host disease (GvHD) following allogeneic stem cell transplantation, and potentially extend overall survival. These advances pave the way for novel microbiome-based therapeutic strategies in hemato-oncology.
- Malard et.al, 2022 – Faecal microbiota transplantation in patients with haematological malignancies undergoing cellular therapies: from translational research to routine clinical practice (The Lancet Haematology)
- Chang, C. C., Hayase, E., & Jenq, R. R. 2021. The role of microbiota in allogeneic hematopoietic stem cell transplantation (Expert Opinion on Biological Therapy)
- Peled et al., 2020 — Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation (New England Journal of Medicine)
- Galloway-Peña et al., 2019 — “Fecal Microbiome, Metabolites, and Stem Cell Transplant Outcomes: A Single-Center Pilot Study” (Open Forum Infectious Diseases)
- Kakihana et al., 2016 — “Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut” (Blood)
- Shono et al., 2016 — Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice (Science Translational Medicine)
- Jenq et al., 2015 — “Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease” (Biology of Blood and Marrow Transplantation)
Early Access Program
- The medicine is intended to treat a serious, rare, or debilitating disease,
- No appropriate treatment is available,
- The medicine is not involved in a clinical trial with human subjects (CTHS) for commercial purposes,
- Or, if the medicine is part of a commercial clinical trial, the patient cannot participate, treatment cannot be delayed, and the company has committed to submitting an early access request,
- The medicine’s efficacy and safety are presumed based on available clinical data.
Clinical trials, clinical data, and scientific publications
Clinical Trials
- MaaT013 – Phase III Clinical Trial – ARES: https://clinicaltrials.gov/study/NCT04769895
- MaaT033 – Phase II Clinical Trial – PHOEBUS: https://clinicaltrials.gov/study/NCT05762211
Clinical Data
- MaaT Pharma – MaaT013 – Phase III Results
- OncLive – ARES Trial of MaaT013 in GI-aGVHD
- Presented data during ASH Congress in December 2024 and at the EHA Congress in June 2025
- To know more about the Early Access Program: EMA – Human Regulatory Overview
Scientific Publications
- Abedin et al., 2021 – Ruxolitinib resistance or intolerance in steroid-refractory acute graft-versus-host disease – a real-world outcomes analysis (British Journal of Haematology)
- Penack O et al.,2021 – Prophylaxis and management of graft-versus-host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation (The Lancet Haematology)
- Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al.(2012) – First and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American society of blood and marrow transplantation. (Biol Blood Marrow Transplant)
- Ferrara JL et al., 2009 – Graft-versus-host disease (The Lancet)
- Malard F et al. (2023) – Pooled allogeneic faecal microbiota MaaT013 for steroid-resistant gastrointestinal acute graft-versus-host disease: a single-arm, multicentre phase 2 trial. (eClinicalMedicine)
- Malard F. et al. (2021) – Restoration of gut microbiota diversity with autologous fecal microbiota transfer in acute myeloid leukemia patients. (Nature communications)
- Malard F. et al. (2018) – High gastrointestinal microbial diversity and clinical outcome in graft-versus-host disease patients (Bone Marrow Transplantation)
- Gefen, R., Dourado, J., Emile, S.H. et al.(2025) – Fecal microbiota transplantation for patients with ulcerative colitis: a systematic review and meta-analysis of randomized control trials. Techniques in Coloproctology – Springer Nature
- Reygner.et al. (2024) – Reduction of product composition variability using pooled microbiome ecosystem therapy and consequence in two infectious murine models (Applied and Environmental Microbiology – ASM Journals)
- Laperrousaz B. et al (2024) – Safety comparison of single-donor and pooled fecal microbiota transfer product preparation in ulcerative colitis: systematic review and meta-analysis. (BMC Gastroenterology)

